
Chemistry, 14.12.2020 20:30, clietmaster112
Gas law A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new pressure? The temperature in the number of moles stay the same.
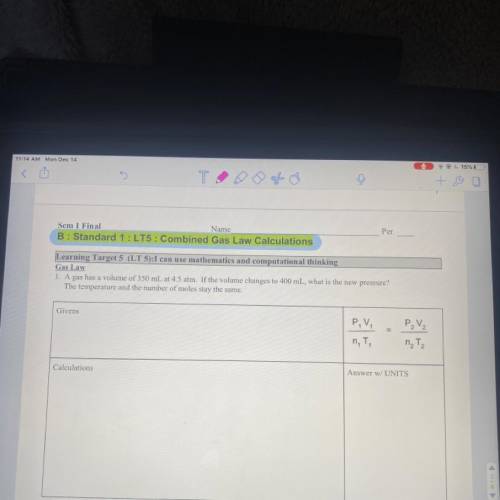

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1

Chemistry, 23.06.2019 05:30, khaylaperry
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
Do you know the correct answer?
Gas law
A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new p...
Questions in other subjects:


Biology, 06.07.2019 00:00

Mathematics, 06.07.2019 00:00



Advanced Placement (AP), 06.07.2019 00:00

History, 06.07.2019 00:00









