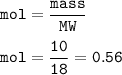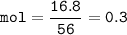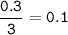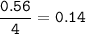Chemistry, 14.12.2020 14:20, praveen35301
3Fe + 4H2O ‐> Fe3O4 + 4H2 10 g of water vapour are passed on 16.8 g of iron which is heated till redness find the limiting reactant

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2


Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
Do you know the correct answer?
3Fe + 4H2O ‐> Fe3O4 + 4H2
10 g of water vapour are passed on 16.8 g of iron which is heated ti...
Questions in other subjects:


English, 23.05.2020 07:02