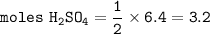Chemistry, 13.12.2020 23:50, muziqbox594
15. Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely with 6.4 moles of lithium hydroxide?
LIOH + H2SO4 -> Li2SO4 + H20 (2 points)
12.8 mol H2SO4
6.4 mol H2SO4
O 3.2 mol H2SO4
O 2.1 mol H2SO4

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
Do you know the correct answer?
15. Upon balancing the equation below, how many moles of sulfuric acid are needed to react completel...
Questions in other subjects:


Biology, 14.10.2019 23:00


History, 14.10.2019 23:00


Mathematics, 14.10.2019 23:00

Mathematics, 14.10.2019 23:00