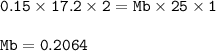A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
Th...

Chemistry, 13.12.2020 23:20, bekahmc1p6k6vj
A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
The student carried out a titration experiment to find the volume of 0.150 mol/dmº
H2SO4 needed to neutralise the KOH.
The student found that, on average, 17.20 cm of the H2SO4 solution was
required for neutralisation.
Calculate the concentration of the KOH solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1


Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Chemistry, 09.09.2020 21:01


Biology, 09.09.2020 21:01


Chemistry, 09.09.2020 21:01

Mathematics, 09.09.2020 21:01