
Chemistry, 13.12.2020 04:40, jadeochoa4466
For the following reaction, calculate how many moles of each product are formed when 4.05 g of water is used. 2H20 →2H2 + O2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 15:30, abelxoconda
How many milliliters of 0.550 m hydriodic acid are needed to react with 25.00 ml of 0.217 m csoh?
Answers: 2

Chemistry, 23.06.2019 16:00, baller1216
Which part of the mantle is similar to the crust ? (science)
Answers: 1

Chemistry, 23.06.2019 16:30, irishvball7
The body of water labeled with the number 3 on the map above is the and the body of water labeled with the number 7 is the a. orinoco river . . atlantic ocean b. orinoco river . . pacific ocean c. paraná river . . pacific ocean d. amazon river . . atlantic ocean
Answers: 3
Do you know the correct answer?
For the following reaction, calculate how many moles of each product are formed when 4.05 g of water...
Questions in other subjects:











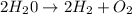
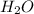 is used to form 2 moles of
is used to form 2 moles of 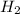 and 1 mole of
and 1 mole of 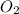
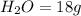 , 1 mole of
, 1 mole of 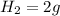 , and 1 mole of
, and 1 mole of 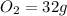 .
.



