Chemistry, 12.12.2020 17:10, janayshas84
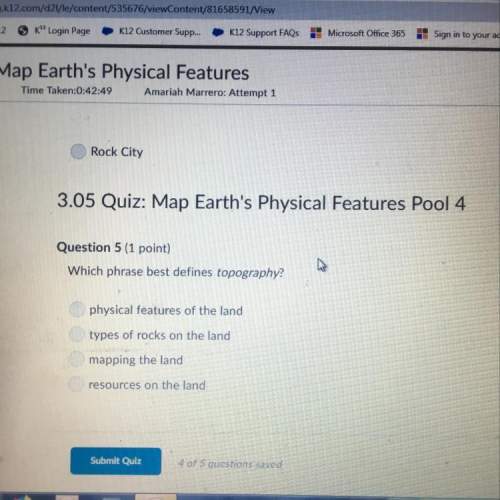
What amount of the excess reagent remains when 0.30 mol NH3 reacts with 0.40 mol O2 to produce NO and H2O? 4NH3 +502 + 4NO + 6H20
(A) 0.10 mol NH,
(B) 0.10 mol
(C) 0.025 mol NH
(D) 0.025 mol O2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
Do you know the correct answer?
What amount of the excess reagent remains when 0.30 mol NH3 reacts with 0.40 mol O2 to produce NO an...
Questions in other subjects:

Mathematics, 21.03.2020 05:28

Mathematics, 21.03.2020 05:28





History, 21.03.2020 05:28


Mathematics, 21.03.2020 05:28


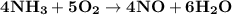
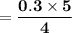
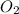
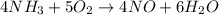
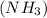 reacts with = 5 moles of oxygen
reacts with = 5 moles of oxygen 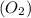
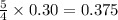 moles of oxygen
moles of oxygen