

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, markmlg122
Two things that biomedical has invented or innvated
Answers: 1



Chemistry, 23.06.2019 01:00, breemills9552
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1
Do you know the correct answer?
A 2.89 g sample of a metal chloride, MCl2, is dissolved in water and treated with excess aqueous sil...
Questions in other subjects:



Biology, 21.07.2019 00:00


History, 21.07.2019 00:00

Mathematics, 21.07.2019 00:00

Mathematics, 21.07.2019 00:00



History, 21.07.2019 00:00


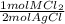 = 0.0224 mol MCl₂
= 0.0224 mol MCl₂



