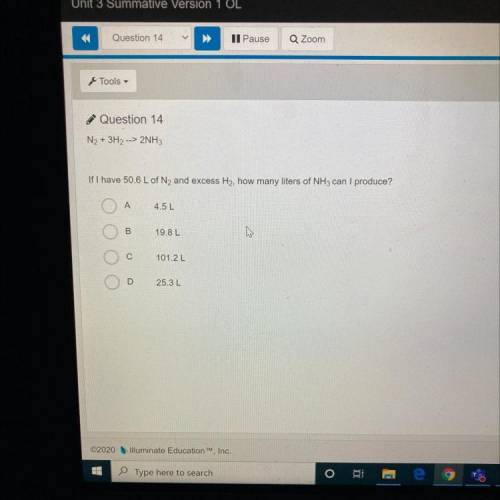
N2 + 3H2, -> 2NH3
If I have 50.6 L of N2 and excess H2, how many liters of NH3 can I produc...

Chemistry, 12.12.2020 16:00, greatsavagebeast
N2 + 3H2, -> 2NH3
If I have 50.6 L of N2 and excess H2, how many liters of NH3 can I produce?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 15:30, ashtonviceoxd21i
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b. colder climates near the equator c. large waves on the cost of europe d. warm climates in northern europe
Answers: 1
Do you know the correct answer?
Questions in other subjects:


History, 28.10.2020 22:00

English, 28.10.2020 22:00


Mathematics, 28.10.2020 22:00


Mathematics, 28.10.2020 22:00

Mathematics, 28.10.2020 22:00


Mathematics, 28.10.2020 22:00






