
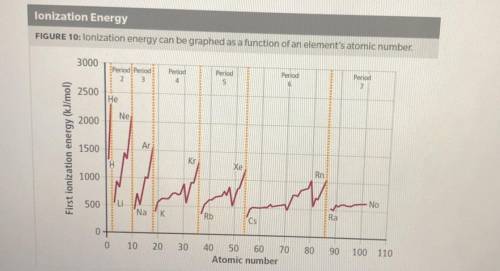
1. How does ionization energy change with atomic number? Use evidence from the graph to support your claim.
2. How does ionization energy change across a period and down a group on the periodic table? Use evidence from the graph to support your claim.
3. What describes an effect on ionization energy when moving down a group? Select all correct answers.
A. The ionization energy increases down a group
B. The ionization energy decreases down a group
C. The valence electrons are in energy levels farther from the nucleus
D. The shielding effect is less

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
Do you know the correct answer?
1. How does ionization energy change with atomic number? Use evidence from the graph to support your...
Questions in other subjects:





Biology, 25.06.2019 18:10

Social Studies, 25.06.2019 18:10



History, 25.06.2019 18:10






