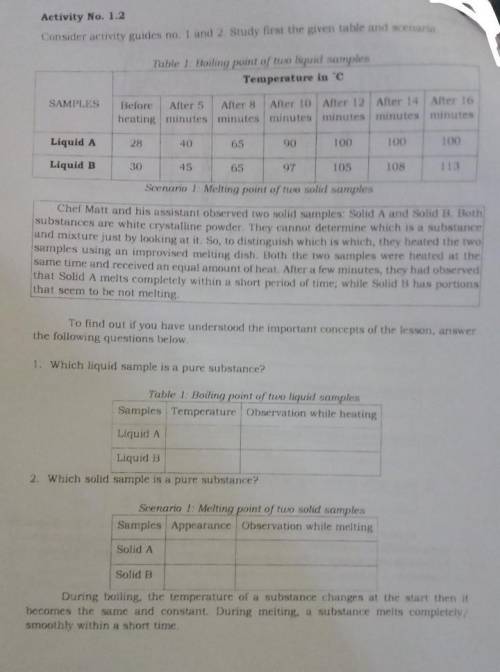
Activity No. 1.2
Consider activity guides no 1 and 2 study first the given table and scenario
...

Activity No. 1.2
Consider activity guides no 1 and 2 study first the given table and scenario
SAMPLES
Table 1 Hoiling point of tuo liquid samples
Temperature in C
Before After 5 After 8 After 10 After 12 After 14 Alter 16
heating minutes minutes minutes minuten minutes minutes
00
100 100 100
Liquid A
07
105
108
Liquid D
Scenario 1 Melting pom of two solid samples
Cher Matt and his assistant observed two solid samples: Solid A and Solid D. Both
substances are white crystalline powder. They cannot determine which is a substance
and mixture just by looking at it. So, to distinguish which is which they heated the two
samples using an improvised melting dish. Both the two samples were heated at the
same time and received an equal amount of heat. Aner a few minutes, they had observed
that Solid A melts completely within a short period of time, while Solid B has portions
that seem to be not melting
To find out if you have understood the important concepts of the lesson, answer
the following questions below
1. Which liquid sample is a pure substance?
Table 1: Boiling point of two liquid samples
Samples Temperature Observation while heating
Liquid A
Liquid B
2. Which solid sample is a pure substance?
Scenano l: Melting point of two solid samples
Samples Appearance Observation while melting
Solid A
Solid B
During boiling, the temperature of a substance changes at the start then it
becomes the same and constant. During melting, a substance melts completely
smoothly within a short time

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Mathematics, 25.06.2019 16:20

History, 25.06.2019 16:20



History, 25.06.2019 16:20







