
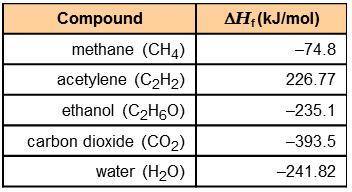
Using the information in the table to the right, calculate the enthalpy of combustion of each of the following substances:
acetylene:
ethanol:
The combustion of 0.25 mol of an unknown organic compound results in the release of 320 kJ of energy. Which of the compounds in the table could be the unknown compound?
ANSWERS:
1.
acetylene: -1,256 kJ/mol
ethanol: -1,277 kJ/mol
2.
ethanol
I already know the answers (they're right there ^) i just need to know HOW you find the enthalpy combustion of acetylene and ethanol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2



Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
Do you know the correct answer?
Using the information in the table to the right, calculate the enthalpy of combustion of each of the...
Questions in other subjects:



Mathematics, 21.08.2019 08:00




Mathematics, 21.08.2019 08:00


History, 21.08.2019 08:10







