
Chemistry, 10.12.2020 20:50, YaBoiMando2061
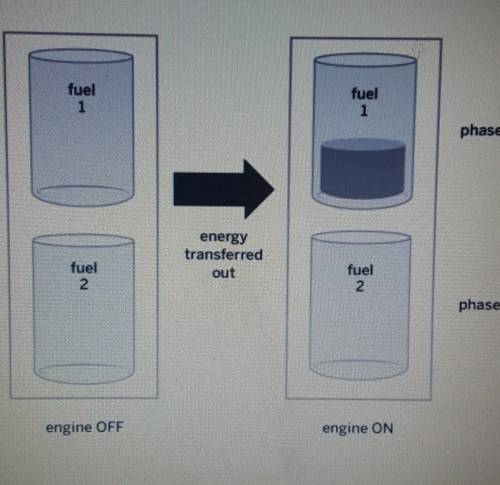
PLEASE ANSWER A certain type of ship has two tanks in its engine. Each tank contains a different type of fuel. When the engine turns on, the same amount of energy is transferred out of both fuels as shown in the diagram below. Why did fuell change phase. but fuel 2 stayed the same? Explain what happened to the molecules of both fuels.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
Do you know the correct answer?
PLEASE ANSWER
A certain type of ship has two tanks in its engine. Each tank contains a different ty...
Questions in other subjects:

Mathematics, 05.12.2021 08:20

Mathematics, 05.12.2021 08:20

Arts, 05.12.2021 08:20

Biology, 05.12.2021 08:20


Mathematics, 05.12.2021 08:20









