
Chemistry, 10.12.2020 17:00, antoinewill05
Your Kid Brother's Hint List
1. Group 15 has elements with increasing atomic radii in the order Y, Kc, Jf. C. Nb, and Xt Y is the smallest and Yt is the largest.
2. In the 7th period, the order of decreasing ionization energy is Hn, E. Tu, Xt. Jc. Rc. By, and N. Hn has the largest ionization energy and N has the smallest.
3. Group 14's elements' ionization energies in decreasing order are Sh. Id, Fm. Lb. Me, and Jc Sh has
the largest ionization energy and Jc has the smallest.
4. In the group with 6 valence electrons, the order of increasing electronegativity is Tu, L, M, V. D, and
A. A is the most electronegative, and Tu is the least.
5. The following elements are in the same period but do not include the noble gas. The order of decreasing
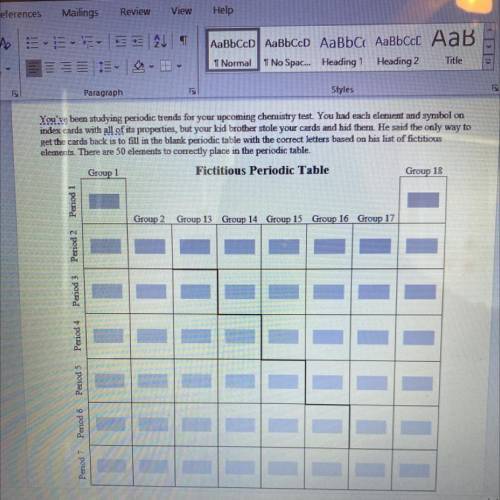

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
Do you know the correct answer?
Your Kid Brother's Hint List
1. Group 15 has elements with increasing atomic radii in the order Y,...
Questions in other subjects:


Social Studies, 26.03.2020 18:42






Mathematics, 26.03.2020 18:42


Mathematics, 26.03.2020 18:43






