B. The reaction is spontaeneous

Chemistry, 10.12.2020 07:10, jescanarias22
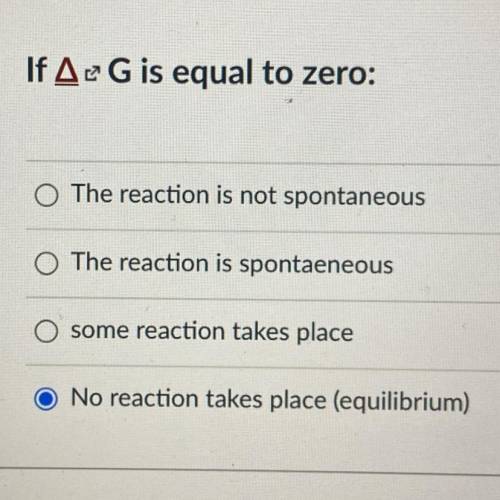
If ΔG is equal to zero:
A. The reaction is not spontaneous
B. The reaction is spontaeneous
C. some reaction takes place
D. No reaction takes place (equilibrium)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
Do you know the correct answer?
If ΔG is equal to zero:
A. The reaction is not spontaneous
B. The reaction is spontaeneous
B. The reaction is spontaeneous
Questions in other subjects:





Biology, 12.01.2020 02:31




Mathematics, 12.01.2020 02:31

Mathematics, 12.01.2020 02:31






