
Chemistry, 10.12.2020 01:40, angelmosby9
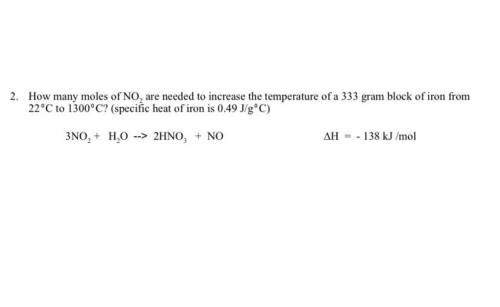
How many moles of NO2 are needed to increase the temperature of a 333 gram block of iron from 220C to 13000C? (specific heat of iron is 0.49 J/g0C) 3NO2 + H2O --> 2HNO3 + NO ∆H = - 138 kJ /mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2
Do you know the correct answer?
How many moles of NO2 are needed to increase the temperature of a 333 gram block of iron from 220C t...
Questions in other subjects:


Mathematics, 17.11.2020 20:10

English, 17.11.2020 20:10



English, 17.11.2020 20:10

Mathematics, 17.11.2020 20:10

Mathematics, 17.11.2020 20:10

Mathematics, 17.11.2020 20:10

Mathematics, 17.11.2020 20:10






