If a container has 8.45 X 1026 atoms of argon. What volume would it occupy at STP?
...

Chemistry, 10.12.2020 01:10, BeeShyanne
If a container has 8.45 X 1026 atoms of argon. What volume would it occupy at STP?
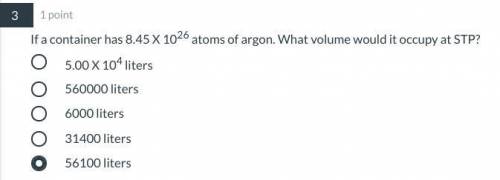

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1


Chemistry, 23.06.2019 06:30, ayoismeisjjjjuan
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 13:00, nellys2096
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2
Do you know the correct answer?
Questions in other subjects:




Health, 23.09.2019 18:20





Geography, 23.09.2019 18:20






