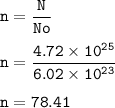Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
Do you know the correct answer?
You have 4.72x10^25 atoms of a substance with MW of 136.94 g/mol How many moles do you have...
Questions in other subjects:

English, 24.11.2020 18:30

Chemistry, 24.11.2020 18:30



Biology, 24.11.2020 18:30





Mathematics, 24.11.2020 18:30