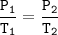Chemistry, 10.12.2020 01:00, chinyere614
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can reach a pressure of 90 psi, the can will explode. To what Temperature must the gases be raised in order for the can to explode? You can assume volume remains constant please help!!!

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 23.06.2019 10:30, krlx
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
Do you know the correct answer?
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can...
Questions in other subjects:

History, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01

Biology, 17.10.2020 06:01

Geography, 17.10.2020 06:01

World Languages, 17.10.2020 06:01