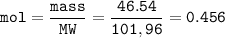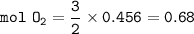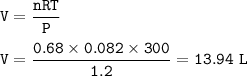Chemistry, 10.12.2020 01:00, haileyhale5
During a laboratory experiment, 46.54 grams of Al2O3 was formed when O2 reacted with aluminum metal at 300.0 K and 1.2 atm. What was the volume of O2 used during the experiment? (5 points) 3O2 + 4Al → 2Al2O3 10.19 liters 11.67 liters 12.51 liters 13.96 liters

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 23.06.2019 19:30, mallorywoods8
(04.03 hc) what type of energy transformation takes place during cellular respiration? use complete sentences to explain how energy is conserved during cellular respiration.
Answers: 1

Chemistry, 23.06.2019 23:20, montgomerykarloxc24x
Agas is inside a cylinder fitted with a piston. the gas and its surroundings are both at 20 ∘c and the gas is compressed as the piston moves and decreases the cylinder volume. the compression takes place slowly enough to allow the gas temperature to stay at 20 ∘c and the work done on the gas by the compression is 2.4 × 103 j.
Answers: 1
Do you know the correct answer?
During a laboratory experiment, 46.54 grams of Al2O3 was formed when O2 reacted with aluminum metal...
Questions in other subjects:



Biology, 29.10.2019 11:31

Social Studies, 29.10.2019 11:31


English, 29.10.2019 11:31