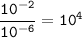Chemistry, 10.12.2020 01:00, elpeke102p73fz3
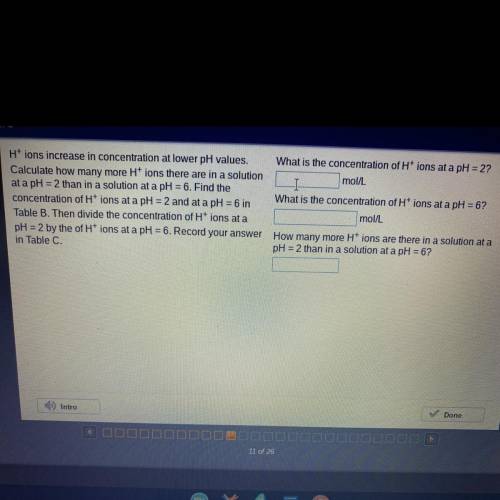
H* ions increase in concentration at lower pH values.
Calculate how many more H" ions there are in a solution
at a pH =2 than in a solution at a pH =6. Find the
concentration of H* ions at a pH =2 and at a pH=6 in
Table B. Then divide the concentration of Ht ions at a
pH =2 by the of H* ions at a pH = 6. Record your answer
in Table C

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
H* ions increase in concentration at lower pH values.
Calculate how many more H" ions there are in...
Questions in other subjects:



Mathematics, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50



Computers and Technology, 23.03.2021 22:50


![\tt \boxed{\bold{pH=-log[H^+]}}](/tpl/images/0967/3115/17f5f.png)
![\tt 2=-log[H^+]\\\\(H^+]=10^{-2}](/tpl/images/0967/3115/2f457.png)
![\tt 6=-log[H^+]\\\\(H^+]=10^{-6}](/tpl/images/0967/3115/40066.png)