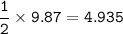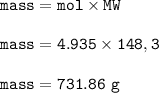Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Do you know the correct answer?
Mg + 2 AgNO₃ → Mg(NO₃)₂ + 2 Ag How many grams of magnesium nitrate, Mg(NO₃)₂, are formed when 9.87 m...
Questions in other subjects:


Computers and Technology, 13.11.2020 19:20





Mathematics, 13.11.2020 19:20


Mathematics, 13.11.2020 19:20