
Chemistry, 08.12.2020 22:40, lopezhailey317
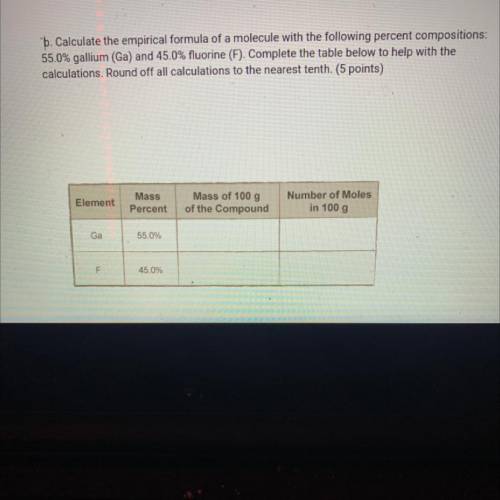
B. Calculate the empirical formula of a molecule with the following percent compositions:
55.0% gallium (Ga) and 45.0% fluorine (F). Complete the table below to help with the
calculations. Round off all calculations to the nearest tenth. (5 points)
Element
Mass
Percent
Mass of 100 g
of the Compound
Number of Moles
in 100 g
Ga
55.0%
F
45.0%

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1


Chemistry, 23.06.2019 12:30, bryantjorell
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1
Do you know the correct answer?
B. Calculate the empirical formula of a molecule with the following percent compositions:
55.0% gal...
Questions in other subjects:



English, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01


English, 02.10.2020 09:01










