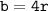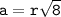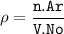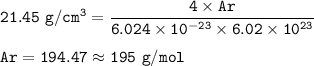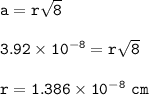Chemistry, 08.12.2020 20:30, nadiarose6345
a metallic object with atoms in a face-centered cubic unit cell with an edge length of 392 pm has a density of 21.45 g/cm^3. calculate the atomic mass and radius of the metal. identify the metal.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1

Do you know the correct answer?
a metallic object with atoms in a face-centered cubic unit cell with an edge length of 392 pm has a...
Questions in other subjects:


Chemistry, 07.10.2021 06:30




Mathematics, 07.10.2021 06:30



Mathematics, 07.10.2021 06:30

Medicine, 07.10.2021 06:30