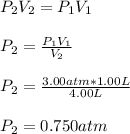A gas is initially confined to a 1.00 L vessel at 3.00 atm of pressure. A valve connecting the 1.00 L vessel to a 3.00 L vessel is opened and the gas expands. What is the final pressure in atm of the gas after the valve is opened, assuming that the volume of the connecting tube is negligible and there is no temperature change?
a. 0.250 atm
b. 0.500 atm
c. 0.750 atm
d. 1.00 atm
e. None of these choices

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, martinez6221
Forests and meadows are often cut down to make way for farms or large number of new homes. what are some of the elements of ecosystems that are lost when plants in these areas are removed?
Answers: 2

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
Do you know the correct answer?
A gas is initially confined to a 1.00 L vessel at 3.00 atm of pressure. A valve connecting the 1.00...
Questions in other subjects:

History, 31.03.2020 18:33


History, 31.03.2020 18:33

Mathematics, 31.03.2020 18:33





Biology, 31.03.2020 18:33

Mathematics, 31.03.2020 18:34