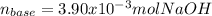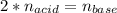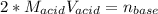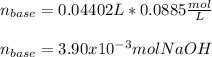Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:50, datboyjulio21
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 07:00, jboii11
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
Do you know the correct answer?
g How many moles of NaOH are present in a sample if it is titrated to its equivalence point with 44....
Questions in other subjects:

Mathematics, 19.03.2021 07:00

History, 19.03.2021 07:00

Mathematics, 19.03.2021 07:00


History, 19.03.2021 07:00




Biology, 19.03.2021 07:00

World Languages, 19.03.2021 07:00