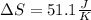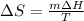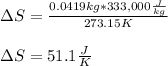Chemistry, 08.12.2020 14:00, choiboiqg5755
Calculate the change in entropy as 0.0419 kg of ice at 273.15 K melts. The latent heat of fusion of water is 333000 J/kg .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
Do you know the correct answer?
Calculate the change in entropy as 0.0419 kg of ice at 273.15 K melts. The latent heat of fusion of...
Questions in other subjects:

Mathematics, 15.06.2021 20:40

Biology, 15.06.2021 20:40

Chemistry, 15.06.2021 20:40



Biology, 15.06.2021 20:40

Mathematics, 15.06.2021 20:40