
Chemistry, 08.12.2020 01:50, 23ricorvan
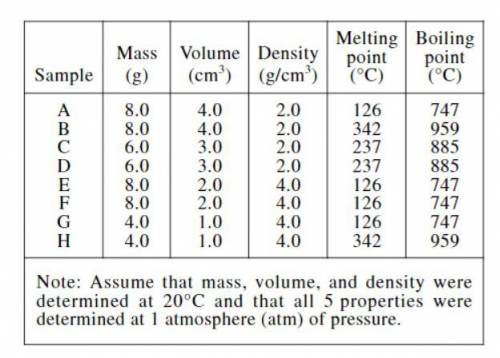
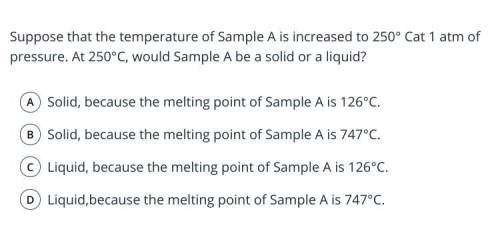
A teacher provided the table below to the students in a science class. The table gives 5 properties for each of Samples A−H. The students were told to assume that each sample is a completely solid cube composed of a single hypothetical pure substance.
The teacher asked each of 4 students to explain how these data could be used to predict which samples are composed of the same substance.
Student 1
If 2 samples have the same values for all 5 properties, they are composed of the same substance. If 2 samples have different values for any of the 5 properties, they are composed of different substances.
Student 2
If 2 samples have the same values for any 3 or more of the 5 properties, they are composed of the same substance. If 2 samples have the same values for fewer than 3 of the 5 properties, they are composed of different substances.
Student 3
If 2 samples have the same mass, volume, and density, they are composed of the same substance. If 2 samples have different values for any of these 3 properties, they are composed of different substances. Neither melting point nor boiling point, by itself, can distinguish between substances.
Student 4
If 2 samples have the same density, melting point, and boiling point, they are composed of the same substance. If 2 samples have different values for any of these 3 proper-ties, they are composed of different substances. Neither mass nor volume, by itself, can distinguish between substances.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Garciaapril1597
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
Do you know the correct answer?
A teacher provided the table below to the students in a science class. The table gives 5 properties...
Questions in other subjects:

Health, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01



Mathematics, 12.10.2020 01:01


Health, 12.10.2020 01:01






