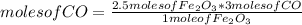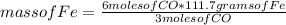Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO2 (g)
How many grams of iron can be produced from 2.5 moles of Fe2O3 and 6.0 moles of CO? Hint: limiting reactant problem
O A. 140 g
B. 335 g
C. 55.858
D. 223 g Fe
E. 279 g Fe

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
Do you know the correct answer?
Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO...
Questions in other subjects:


Mathematics, 19.02.2020 23:29



Mathematics, 19.02.2020 23:29

Mathematics, 19.02.2020 23:29

SAT, 19.02.2020 23:29