

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
Do you know the correct answer?
Ammonia NH3 gas and oxygen O2 gas react to form nitrogen N2 gas and water H2O vapor. Suppose you hav...
Questions in other subjects:

Mathematics, 15.06.2021 18:20

Mathematics, 15.06.2021 18:20



Mathematics, 15.06.2021 18:20






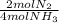 = 1.0 mol N₂
= 1.0 mol N₂



