
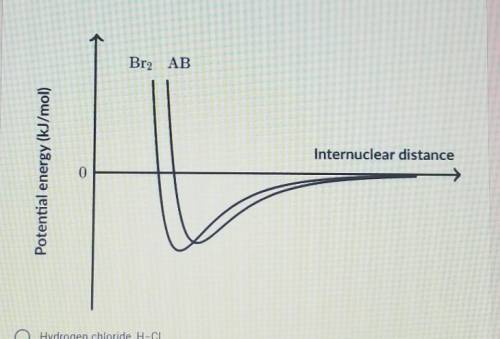
The graph below shows the potential energy as a function of internuclear distance for molecular bromine, Br-Br and an unknown heteronuclear diatomic molecule, A-B. Based on the data in the graph, which of the following correctly identifies the diatomic molecule A-B?
A.) Hydrogen Chloride, H-Cl
B.) Carbon Monoxide, C=_O (Triple Bond)
C.) Sulfur Monoxide, S=O (Double Bond)
D.) Iodine Monobromide, I-Br

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
Do you know the correct answer?
The graph below shows the potential energy as a function of internuclear distance for molecular brom...
Questions in other subjects:







Mathematics, 01.10.2019 20:20

Mathematics, 01.10.2019 20:20







