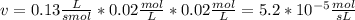Chemistry, 18.08.2019 12:00, emiliapizzillo
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are both 0.020 moles per liter and k = 1.3 × 10-1 m-1s-1, what is the reaction rate?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
Do you know the correct answer?
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are bo...
Questions in other subjects:

Mathematics, 15.09.2020 01:01

History, 15.09.2020 01:01

History, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

History, 15.09.2020 01:01

English, 15.09.2020 01:01

English, 15.09.2020 01:01

History, 15.09.2020 01:01

English, 15.09.2020 01:01