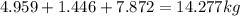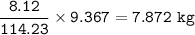Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Do you know the correct answer?
A mixture of hydrocarbons contains 34.8% heptane, C7H16, 10.1% nonane, C9H20, and 55.1% octane, C8H1...
Questions in other subjects:


English, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00





History, 14.04.2021 14:00

Law, 14.04.2021 14:00

Biology, 14.04.2021 14:00


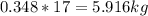 Mass C in Heptane (MW=100.21 g/mol) :
Mass C in Heptane (MW=100.21 g/mol) :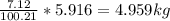
 Mass C in Nonane (MW=128.2 g/mol) :
Mass C in Nonane (MW=128.2 g/mol) :
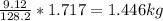
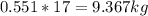 Mass C in Otane (MW=114.23 g/mol) :
Mass C in Otane (MW=114.23 g/mol) :