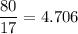Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mimithurmond03
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 21.06.2019 21:30, gallegosarmanni
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1


Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
Do you know the correct answer?
In a lab experiment 80.0 g of ammonia [NH3] and 120 g of oxygen are placed in a reaction vessel. At...
Questions in other subjects:

English, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Chemistry, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00




Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00