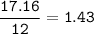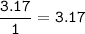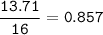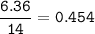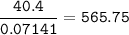Chemistry, 06.12.2020 05:00, timithythaxton
A 40.4 g sample of a protein contains 17.16 g of carbon, 3.17 g of hydrogen, 13.71 g of oxygen, and the rest being nitrogen. This 40.4-gram sample is known to be 0.07141 moles. Determine the molecular formula of this protein.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 23.06.2019 00:00, scottykinkade7860
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
Do you know the correct answer?
A 40.4 g sample of a protein contains 17.16 g of carbon, 3.17 g of hydrogen, 13.71 g of oxygen, and...
Questions in other subjects:




Mathematics, 09.04.2021 19:20


History, 09.04.2021 19:20