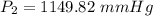Chemistry, 06.12.2020 02:40, live4dramaoy0yf9
What is the final pressure, in mmHg, for a gas in an aerosol can at an initial pressure of 1.40 atm at 12°C which is heated to 35 °C?
O a. 985 mmHg
O b. 1150 mmHg
O c. 980 mmHg
d. 1.1 x 10^3 mmHg

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 05:00, xxaurorabluexx
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
Do you know the correct answer?
What is the final pressure, in mmHg, for a gas in an aerosol can at an initial pressure of 1.40 atm...
Questions in other subjects:



Mathematics, 26.01.2022 14:00

English, 26.01.2022 14:00


Mathematics, 26.01.2022 14:00


Mathematics, 26.01.2022 14:00

Mathematics, 26.01.2022 14:00


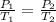
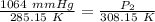 Cross-multiply:
Cross-multiply: 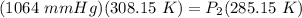 Multiply:
Multiply: 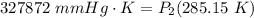 Isolate P₂:
Isolate P₂: 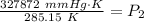 Divide:
Divide: 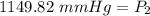 Rewrite:
Rewrite: