Chemistry, 09.12.2019 05:31, wednesdayA
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00-l container. this is the equation: co(g)+h2o(> < -- co2(g) + h2(g). how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, Unstinct
Lithium diisopropylamide [(ch3)2ch]2nli, referred to as lda, enjoys many uses as a strong base in synthetic organic chemistry. it is customarily prepared by the reaction of diisopropylamine [(ch3)2ch]2nh with butyllithium. draw the products of the reactions in the appropriate boxes and select the acid, base, conjugate acid, and conjugate base. be sure to answer all parts.
Answers: 2

Chemistry, 21.06.2019 17:30, brasherfamily14
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1
Do you know the correct answer?
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions in other subjects:



Health, 08.04.2021 18:50

English, 08.04.2021 18:50

English, 08.04.2021 18:50




Mathematics, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50


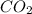 added will be 1.12 mole.
added will be 1.12 mole. and
and 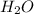 at equilibrium = 0.200 mol
at equilibrium = 0.200 mol at equilibrium = 0.600 mol
at equilibrium = 0.600 mol at equilibrium.
at equilibrium.



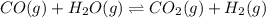
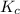 will be,
will be,![K_c=\frac{[H_2][CO_2]}{[CO][H_2O]}](/tpl/images/0409/6473/e1151.png)