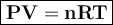Chemistry, 05.12.2020 04:10, alayciaruffin076
A sealed 5.00L flask contains an unknown gas at 1.05 atm and 296 K. How many moles of the unknown gas are in the flask?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
Do you know the correct answer?
A sealed 5.00L flask contains an unknown gas at 1.05 atm and 296 K. How many moles of the unknown ga...
Questions in other subjects:

Mathematics, 04.11.2020 18:30



Physics, 04.11.2020 18:30

Biology, 04.11.2020 18:30


Mathematics, 04.11.2020 18:30

Mathematics, 04.11.2020 18:30