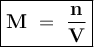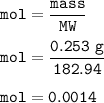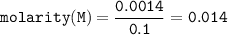Chemistry, 05.12.2020 03:10, Leggett8152
A chemist prepares a solution by adding 253 mg of Co(NO3)2 (MW = 182.94 g/mol ) to a volumetric flask, and then adding water until the total volume of the contents of the flask reaches the calibration line that indicates 100 mL . Determine the molarity of the prepared solution.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
Do you know the correct answer?
A chemist prepares a solution by adding 253 mg of Co(NO3)2 (MW = 182.94 g/mol ) to a volumetric flas...
Questions in other subjects:

Computers and Technology, 01.11.2021 14:00


English, 01.11.2021 14:00


Mathematics, 01.11.2021 14:00

History, 01.11.2021 14:00

Mathematics, 01.11.2021 14:00

Mathematics, 01.11.2021 14:00

Mathematics, 01.11.2021 14:00