
Chemistry, 04.12.2020 23:30, lizbeth232001
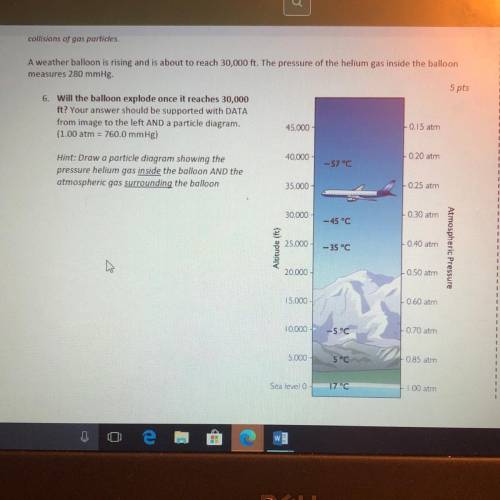
A weather balloon is rising and is about to reach 30,000 ft. The pressure of the helium gas inside the balloon
measures 280 mmHg.
5 pts
6. Will the balloon explode once it reaches 30,000
ft? Your answer should be supported with DATA
from image to the left AND a particle diagram.
45.000
0.15 atm
(1.00 atm = 760.0 mmHg)
40.000
L020 atm
-57°C
Hint: Draw a particle diagram showing the
pressure helium gas inside the balloon AND the
atmospheric gas surrounding the balloon
35.000
025 atm
30.000
0.30 atm
- 45°C
25.000
0.40 atin
Altitude (ft)
- 35 °C
Atmospheric Pressure
20.000
0.50 atm
15.000
0.60 am
10.000
-5°C
070 at
5.000
5°C
0 85 atm
Sea level
17°C
00 am

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 03:30, jennelledenise
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
Do you know the correct answer?
A weather balloon is rising and is about to reach 30,000 ft. The pressure of the helium gas inside t...
Questions in other subjects:

Mathematics, 30.10.2020 19:10


English, 30.10.2020 19:10


History, 30.10.2020 19:10

Mathematics, 30.10.2020 19:10


Chemistry, 30.10.2020 19:10


Mathematics, 30.10.2020 19:10






