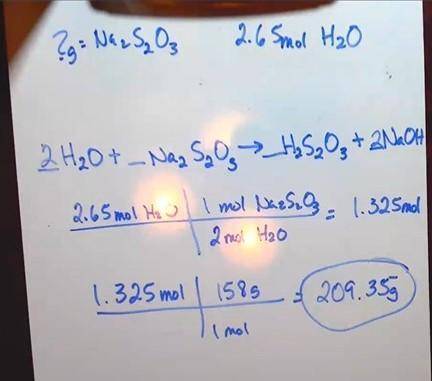Chemistry, 04.12.2020 22:10, aljalloh94
PLS HELP! I CAN"T DO THIS! I AM ABOUT TO CRY COZ NO ONE WILL ANSWER TnT
Chlorine is used by textile manufacturers to bleach cloth. Excess chlorine is destroyed by its reaction with sodium thiosulfate, Na 2 S 2 O 3 :
Na 2 S 2 O 3(aq) + 4Cl 2(g) + 5H 2 O (aq) 2NaHSO 4(aq) + 8HCl (aq)
1. How many grams of Na 2 S 2 O 3 are needed to react with 2.65 mol of H 2 O? (3
marks)
2. How many mol of HCl can form from 25.2 mol of Na 2 S 2 O 3 ? (2 marks)
3. How many Liters of Cl 2 are required to produce 15.7 moles of NaHSO 4 ? (2
marks)
4. How many molecules of HCl can form from 4.92 grams of H 2 O? (3 marks)
I also need the steps. Please and thank you!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
Do you know the correct answer?
PLS HELP! I CAN"T DO THIS! I AM ABOUT TO CRY COZ NO ONE WILL ANSWER TnT
Chlorine is used by textile...
Questions in other subjects:

History, 19.09.2019 21:00

Spanish, 19.09.2019 21:00

Social Studies, 19.09.2019 21:00

Biology, 19.09.2019 21:00




English, 19.09.2019 21:00