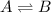Chemistry, 13.01.2020 23:31, chloeethoma24
Which statement correctly defines dynamic equilibrium? at dynamic equilibrium, the rates of forward and reverse reactions are equal. at dynamic equilibrium, the rate of the forward reaction is higher than the rate of the reverse reaction. at dynamic equilibrium, the forward and reverse reactions stop. at dynamic equilibrium, the concentrations of reactants and products are equal.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 08:00, ira51
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
Do you know the correct answer?
Which statement correctly defines dynamic equilibrium? at dynamic equilibrium, the rates of forward...
Questions in other subjects:



History, 03.11.2019 16:31

Mathematics, 03.11.2019 16:31

Biology, 03.11.2019 16:31

English, 03.11.2019 16:31

Health, 03.11.2019 16:31


Physics, 03.11.2019 16:31