
Chemistry, 04.12.2020 01:00, kevonmajor
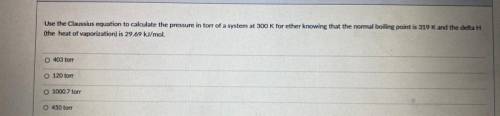
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing that the normal boiling point is 319 K and the delta H
the heat of vaporization) is 29.69 kl/mol
403 ton
120 ton
1000 7 ore
450 for

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1
Do you know the correct answer?
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing t...
Questions in other subjects:

Chemistry, 08.12.2020 01:40

History, 08.12.2020 01:40


Chemistry, 08.12.2020 01:40

Chemistry, 08.12.2020 01:40



Computers and Technology, 08.12.2020 01:40








