
Chemistry, 03.12.2020 18:10, fernandaElizondo
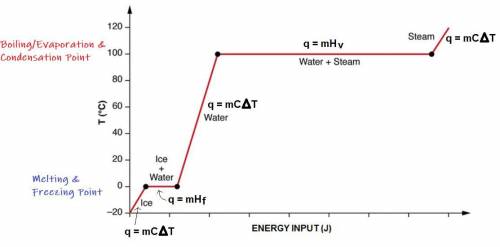
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. Expected Answer = 501,440 J Before trying to solve this problem, explain:
what is happening to the water from 60.0 degrees Celsius to 100.0 degrees Celsius?
what happens at 100.0 degrees Celsius?
what happens from 100.0 degrees Celsius to 140.0 degrees Celsius?
Then solve the full problem, showing work & units.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 09:20, goldwinner300
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2

Chemistry, 23.06.2019 09:30, sharmadaman641
What is the best describtion of the side of the moon that faces earth?
Answers: 2
Do you know the correct answer?
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. E...
Questions in other subjects:

Mathematics, 16.04.2020 20:23





Mathematics, 16.04.2020 20:23



Mathematics, 16.04.2020 20:23






