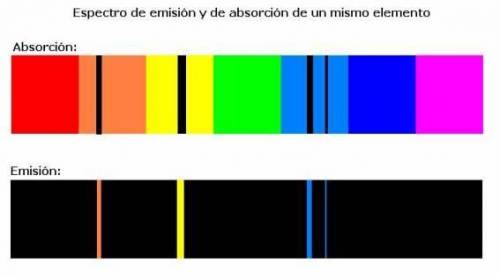
Chemistry, 03.12.2020 07:00, alexreddin3127
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not quantizied
a. lines would be shifted into the ultraviole region
b. there would be fewer lines
c. there will be more lines
d. the spectrum would be constnuous

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2


Do you know the correct answer?
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not q...
Questions in other subjects:


History, 17.09.2019 13:50

Mathematics, 17.09.2019 13:50






Mathematics, 17.09.2019 13:50

Chemistry, 17.09.2019 13:50