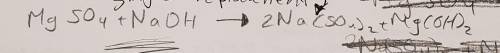Chemistry, 02.12.2020 21:30, irvinbhangal2
3. Magnesium sulfate is added to sodium hydroxide to produce sodium sulfate and magnesium hydroxide (10 points)
a. Write the skeletal equation and Balance the chemical equation describing the reaction above.
b. What kind of reaction is this?
c. Rewrite the balanced chemical equation, adding state symbols for all compounds in the reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1



Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
Do you know the correct answer?
3. Magnesium sulfate is added to sodium hydroxide to produce sodium sulfate and magnesium hydroxide...
Questions in other subjects:





Computers and Technology, 01.02.2020 09:43



Mathematics, 01.02.2020 09:43

Biology, 01.02.2020 09:43