PLEASE HELP
give the electron configuration of vanadium (V), atomic number 23
Give the...

PLEASE HELP
give the electron configuration of vanadium (V), atomic number 23
Give the noble gas configuration of vanadium (V), atomic number 23
List the energy levels for the orbital configuration of vanadium (V), atomic number 23
How does the atomic radius change going down and across the periodic table?
How does the first ionization energy change going down and across the periodic table?
How does electronegativity change going down and across the periodic table?
How does the radius of a positive and negative ion compared to a neutral atom?
Match: A. Ionic bond B. Covalent bond C. Metallic bond __sharing of electrons __freely moving electrons __transfer electrons
Describe the dipole dipole force
Describe hydrogen bonding
Describe the Van der waals forces
Imagine you need to take a medicine that the doctor has prescribed for you. Explain why scientist who developed that medicine would need to know whether or not the compound in that medicine is polar. How might a polar medicine behave differently within your body than a non-polar medicine word? (Answer in 1 to 2 paragraphs)
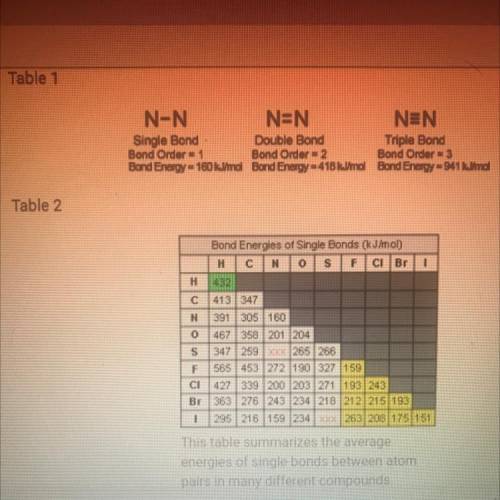
According to table 2, which is the strongest bond? Which is the week is fine? Based on what you know about the atomic radii and electronegativity of the elements involved in the bonds, why do you think these to have the most extreme bond-energy values?
How are the bond energies of each bond listed in table two determined?
Why do you think there aren’t bond energy values given in table 2 for N-S and S-I?
Based on tables one and two how would you describe the trend in bond strength of single double and triple bonds?
Based on table 2, how would you describe the trend in strength of bonds formed by the elements carbon nitrogen and oxygen? Would you describe this trend as a periodic trend? Why or why not?
What is the VSEPR theory?
How does electron repulsion determine molecular shape?
How do lone electron pairs affect molecular shape?
Draw the Lewis structure for the Se and 2 H atoms
Draw the Lewis structure for the SeH(2) molecule
What shape would SeH(2) have? Draw the molecule
Identify each of the following as a covalent compound, or ionic compound. Then provide either the formula for compounds identified by name or the name for those identified by formula:
Li(2)O
Dibitrogen trioxide
PCl(3)
Manganese(|||) oxide
Calcium bromide
THANK YOU FOR YOUR HELP <3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
Do you know the correct answer?
Questions in other subjects:



Biology, 26.04.2021 05:20

Mathematics, 26.04.2021 05:20

Mathematics, 26.04.2021 05:20



Geography, 26.04.2021 05:30

Mathematics, 26.04.2021 05:30

Biology, 26.04.2021 05:30






