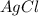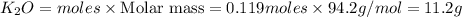Chemistry, 01.12.2020 07:30, ijustneedhelp29
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reagent and what is the theoretical yield of the reaction?
Hint: write the balanced reaction
K - 39.10 g/mol
O - 15.999 g/mol
ANSWER CHOICES:
A.) O2 is limiting, 11.2 g of K2O formed
B.) K is limiting, 14.7 g of K2O formed
C.) K is limiting, 11.2 g of K2O formed
D.) O2 is limiting, 14.7 g of K2O formed
E.) O2 is limiting, 19.2 g of K2O formed

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 22:30, safiyabrowne7594
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
Do you know the correct answer?
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reag...
Questions in other subjects:


English, 15.04.2020 19:20

Social Studies, 15.04.2020 19:20


Mathematics, 15.04.2020 19:20



History, 15.04.2020 19:20

World Languages, 15.04.2020 19:20

Mathematics, 15.04.2020 19:21


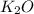 formed
formed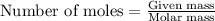
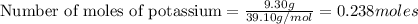
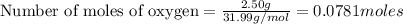
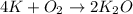
 require 1 mole of
require 1 mole of 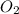
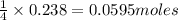 of
of 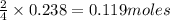 of
of