
Chemistry, 30.11.2020 17:40, williamnason123
Which reactions have a positive ΔSrxn? 2A(g)+B(g)⟶4C(g) 2A(g)+3B(g)⟶4C(g) A(s)+2B(g)⟶C(g) 2A(g)+B(s)⟶3C(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1


Chemistry, 23.06.2019 06:00, kelyanthecrafte
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1

Chemistry, 23.06.2019 09:00, sammypaige08
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
Do you know the correct answer?
Which reactions have a positive ΔSrxn? 2A(g)+B(g)⟶4C(g) 2A(g)+3B(g)⟶4C(g) A(s)+2B(g)⟶C(g) 2A(g)+B(s)...
Questions in other subjects:

Mathematics, 17.05.2021 22:00





Mathematics, 17.05.2021 22:00



Mathematics, 17.05.2021 22:00

Mathematics, 17.05.2021 22:00


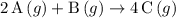 (the first choice.)
(the first choice.)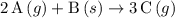 (the fourth choice.)
(the fourth choice.) 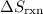 denotes the entropy change in a reaction.
denotes the entropy change in a reaction. 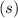 is the state symbol for solids, whereas
is the state symbol for solids, whereas 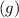 is the state symbol for gases.
is the state symbol for gases.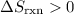 for this reaction.
for this reaction.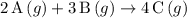 .
. 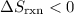 for this reaction.
for this reaction.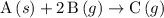 .
. 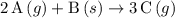 .
. 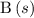 is not a gas.)three gas particles among the products. The number of gas particles that this reaction produces is larger than the number of gas particles that it consumes. Therefore, it is likely that
is not a gas.)three gas particles among the products. The number of gas particles that this reaction produces is larger than the number of gas particles that it consumes. Therefore, it is likely that 



