

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
Do you know the correct answer?
0.22g of carbon dioxide are dissolved in 400 cm3 of pure water.
Calculate the concentration in mol/...
Questions in other subjects:

Biology, 19.06.2021 08:00

Biology, 19.06.2021 08:00



Medicine, 19.06.2021 08:00


Arts, 19.06.2021 08:00




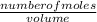
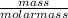
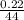 = 0.0005mol
= 0.0005mol 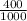 = 0.4dm³
= 0.4dm³ 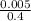 = 0.0125mol/dm³
= 0.0125mol/dm³




