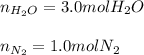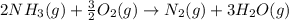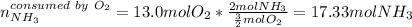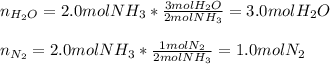Chemistry, 29.11.2020 15:20, westes0376
Ammonia gas(NH3) and oxygen(O2) gas react to form nitrogen gas and water vapor. Suppose you have 2.0 mol of and 13.0 mol of O2 in a reactor. Calculate the largest amount of that could be produced. Round your answer to the nearest .

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
Do you know the correct answer?
Ammonia gas(NH3) and oxygen(O2) gas react to form nitrogen gas and water vapor. Suppose you have 2.0...
Questions in other subjects:


History, 10.12.2021 14:00


Mathematics, 10.12.2021 14:00

Mathematics, 10.12.2021 14:00

Mathematics, 10.12.2021 14:00

History, 10.12.2021 14:00